BioSafety Cabinet Maintenance California
Having the proper maintenance plan in place when it comes to research laboratories and facilities is critical. Its vital that the laboratory workers and surrounding equipment is protected fom pathogens and decontaminants, which is what a Biosafety Cabinet provides. A HEPA filter helps remove the harmful bacteria from all exhausted air exiting the biosafety cabinet. The GL-Tec team can inspect the biosafety cabinets being used in your research laboratories or facilities, and create a maintenance plan to the equipment up to industry standards such as NSF Annex 7. GL-Technologies has become a trusted provider of biosafety cabinet certifications and maintenance programs for organizations both large and small.
To speak with the experts about your biosafety cabinet needs, please fill out our online form or give us a call!
Routine Maintenance Schedule and Repair
As any laboratory or medical research facility will agree, routine biosafety cabinet maintenance can prvent contamination. GL-Technologies supplies routin biosafety cabinet maintenance, repairs and filter changes for all biosafety manufacturing types. Our maintenance program can include:
Weekly, monthly, or yearly decontamination of surfaces
Bi-Annual Full Decontamination
Full Decontamination Between Operational Changes
Decontamination for Building or Facility Relocation
Filter Replacements
Safety Cabinet Repairs
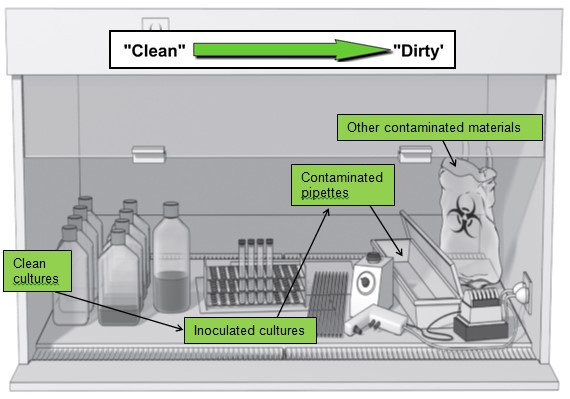
The recommendation by NSF 49 iis that certification is done at a minimum, every 12 months. Of course, there are many organizations and laboratories that re-certify more frequently such as a pharmacy that re-certifies every 6 months as required by USP CHapter <797> on sterile compounding.
California BioSafety Cabinet Tests
jTo verify biosafety cabinets are working properly, there are a variety of tests GL-Technologies will perform. The results need to correlate to the value obtained by NSF for type testing of that particular manufacturer make, model, and size.
The values will be provided by the manufacturer:
Downflow velocity test
Inflow velocity test
Airflow smoke pattern tests
HEPA filter leak test
Cabinet integrity test
Alarm function verification
Blower interlock
Exhaust system performance (for any cabinet connected to the building exhaust system)
Our team understands the role biosafety cabinets play in laboratory, research, and pharmaceutical facilities in keeping contamination in check. Companies both large and small rely on GL-Technologies for certification, maintenance and repair needs!
About GL Technologies
GL Technologies, based in San Diego, is a specialized service provider catering to the highly regulated industries of biopharmaceuticals, pharmaceuticals, medical devices, and government sectors. The company focuses on delivering expert solutions in equipment calibration, validation, and compliance services, ensuring that clients meet stringent GMP (Good Manufacturing Practice) and FDA regulations. GL technologies is a trusted partner from commissioning new plants to decommissioning with compliance. GL can place dedicated motivated quality personnel on site anywhere. A program can be designed or revamped for the customers needs from design of CMMS to SOP development, specification development and performance of calibrations.
With a dedicated team of 29 technicians, GL Technologies offers precision calibration, preventative maintenance, and qualification services for laboratory and production equipment used in critical manufacturing and research processes. The company’s expertise is supporting its clients in maintaining regulatory compliance and operational efficiency.
As a full-service company specializing in equipment calibration, repair, and certification services for biopharmaceutical, pharmaceutical, and medical device industries. Our team has extensive experience working with sPRT calibrations along with CMMS software, HPLC OQ validation, and fume hood certifications. Companies of all sizes rely on our team to implement, maintain, and keep their research and manufacturing processes compliant with regulatory standards. Other specialties include building maintenance systems, and mass spectrometry calibrations. GL Tec specializes in IQ OQ PQ services for clients throughout San Diego, San Francisco, Los Angeles, Orange County, and Riverside!
To speak with the experts about your biosafety cabinet needs, please fill out our online form or give us a call!

Trending Articles
Troubleshooting HPLC Systems in Pharmaceutical Settings
High-Performance Liquid Chromatography (HPLC) is a common technique in pharmaceutical analysis, providing precise separation and quantification of compounds. However, like any analytical method, HPLC systems can encounter issues that impede their functionality. In a pharmaceutical setting where accuracy and reliability are vital, quick and effective troubleshooting is important to maintain productivity and maintain the quality of results.
Streamlining Operations: The Biopharma ERP Advantage
For biopharmaceutical and pharmaceutical organizations, where every moment counts in the race to develop life-saving treatments, efficiency is of the utmost requirement. Biopharma companies face unique challenges, from strict regulatory requirements to complex manufacturing processes. In such a highly regulated and competitive industry, any edge in operational efficiency can make a significant difference. This is where Enterprise Resource Planning (ERP) systems tailored for biopharma play an important role.
To request a quote from GL-Tec, please fill out our online form and we will get back to you promptly.
CLICK HERE TO BEGIN

