The biopharmaceutical industry plays an important role in developing and producing life-saving drugs and therapies. Maintaining the quality and safety of these products is of utmost importance. ...
100+ Sites.
FDA/FDB/EU/Asian
audit
Core Capabilities
Programs engineered around ISO 17025 and cGMP
Asset Management
Lifecycle strategy, criticality, SOPs, metrology hierarchies, audit trails and KPI dashboards
- RCM based asset programs
- Change control
On-Site Technicians
Embedded calibration maintenance teams with high retention and low discipline
- 100+ placements to date
- Lower turnover faster ramp
- PPE ready
Calibration, Validation & More
CMMS, metrology labs, passivation, water testing, TEB III repair
- Calibration & IPT programs
- ISO 17025 accredited lab
- cGMP SOP
Mission
A California based Calibration/ Metrology company specializing in servicing the Biopharmaceutical and Medical Device industries with FDA cGMP compliance, product efficacy, lot repeatability, safety of patients, utilities and manufacturing equipment repeatability, and safety of personnel.
ISO/IEC 17025:2017 & ANSI/NCSL Z540-1-1994 SO Certificate
CLICK HERE FOR ISO/IEC 17025:2017 & ANSI/NCSL Z540-1-1994 SO Certificate
Equipment Calibration, Asset Management Systems, and Validation
Calibration, Repair, Preventive Maintenance, Qualifications, Validations, IQ, OQ, PQ

Equipment Calibration Services
Whether addressing emergency situations, performing routine maintenance, or installation of new equipment , our customers rely on the expertise and diverse experience that GL Technologies LLC can provide. Our experience performing cGMP compliant calibrations and maintenance has been relied upon by industry leaders in the medical device, biopharmaceutical and life science industries as well as local municipalities and military installations.
We specialize in calibration and troubleshooting of Clean Utilities, Automated Process Equipment, Analytical Laboratory Equipment, HVAC controls, SCADA and Building Management Systems and Passivation.
GL Technologies Calibration Services

GL Technologies is a full service calibration provider specializing in the Biopharmaceutical and Medical Device industries. Our management has worked in, managed, and developed FDA cGMP fully compliant programs. Our services include roving crews for the Biopharmaceuticals for regular scheduled calibrations or shutdown and new system situations. GL Technologies also places qualified individuals on various large and small Biopharmaceutical and Medical Device companies. The services we provide include general Calibration/ Metrology support, asset and CMMS management, compliant program development, SOP development, and maintenance/ facilities support.
GL Technologies can fully setup a compliant program including method development, Identification of items to calibrate in a facility or on a system, tolerance assignment, calibration cycle determination, and SOP design. Our management has personally been audited by and developed programs that have been audited by the FDA, FDB, European, and Asian Regulatory Authorities. We specialize in the placement of personnel on sites and are often used to vet potential new hires for Biopharmaceutical and Medical Device companies. GL Technologies has placed over 180 individuals at sites in California and abroad. There are no fees or penalties for the full time hiring of these individuals. Most of the current CA Biopharmaceutical programs are now managed and staffed by former GL team members.
Equipment Calibration Articles
Calibration Software

Hood Certification (Fume & Bio Safety Cabinets
Analytical Services
(HPLC PM OQ Repair, Sartorious)
sPRT Calibration (Standard Resistance Thermometer)
Building Maintenance Systems
CMMS Software
Fume Hood Certification
Join the GL Technologies Team!
For some of us, GL Technologies is the best first job they can have. For others, this is their career-long home. And there are many more in between. Whatever your career goals, if you work with integrity and committment there’s a place for you!
IQ OQ PQ Services
The team at GL Technologies provides IQ OQ PQ services for biopharmaceutical and pharmaceutical clients throughout California to implement theses processes in to their organization. A company’s commitment to IQ OQ PQ qualifications is of the utmost importance. IQ focuses on verifying proper equipment installation and documentation, making sure there is a solid foundation for subsequent operations. OQ dives into the equipment’s functionality, confirming that it operates within defined specifications under various conditions. Finally, PQ assesses the system’s performance by subjecting it to real-world scenarios, verifying it consistently meets the desired outcomes and regulatory requirements.


Mass Spec Calibrations
Biopharmaceutical and pharmaceutical companies throughout California rely on the team at GL Technologies for their mass spectrometry calibration needs. Mass spectrometry is a critical process in analytical chemistry that verifies the accuracy and precision of mass spectrometers, which are vital tools for identifying and quantifying chemical compounds in various samples. Calibration involves adjusting the instrument’s settings and parameters to align the measured “mass-to-charge (m/z)” of ions with their known values. We achieve this alignment by introducing a known standard compound into the mass spectrometer and adjusting its parameters until the measured mass spectra match the expected values. For biopharma and pharmaceutical organizations, proper calibration not only increases the reliability of analytical results but also allows for the detection of subtle differences in molecular masses, making it indispensable in these industries.
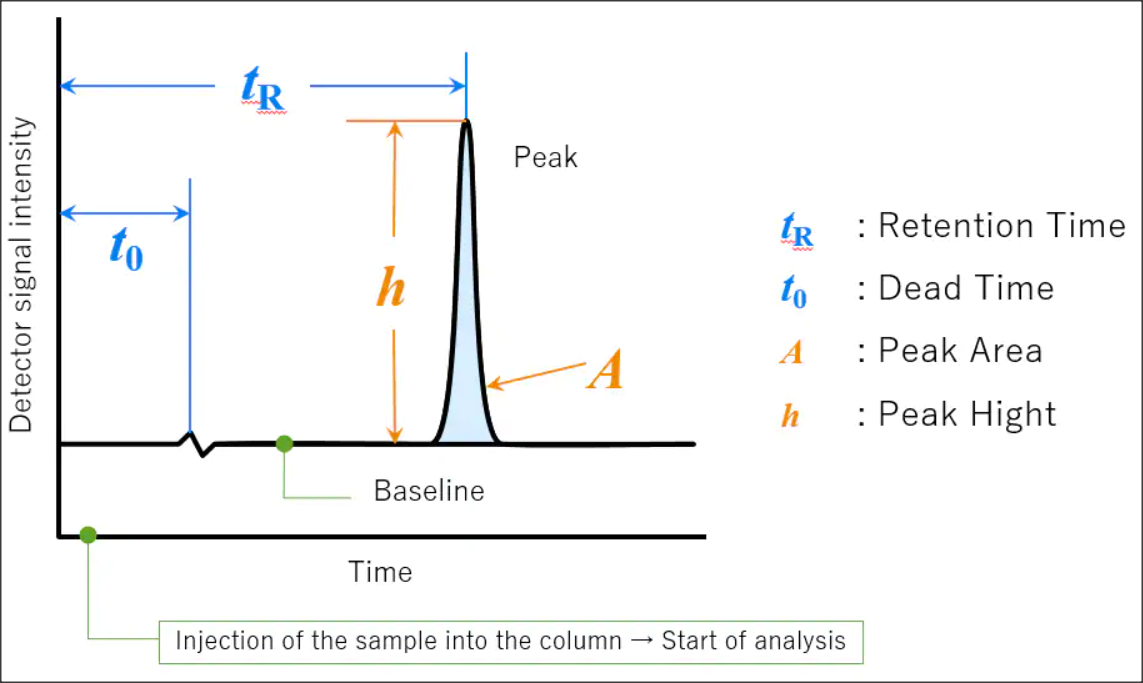
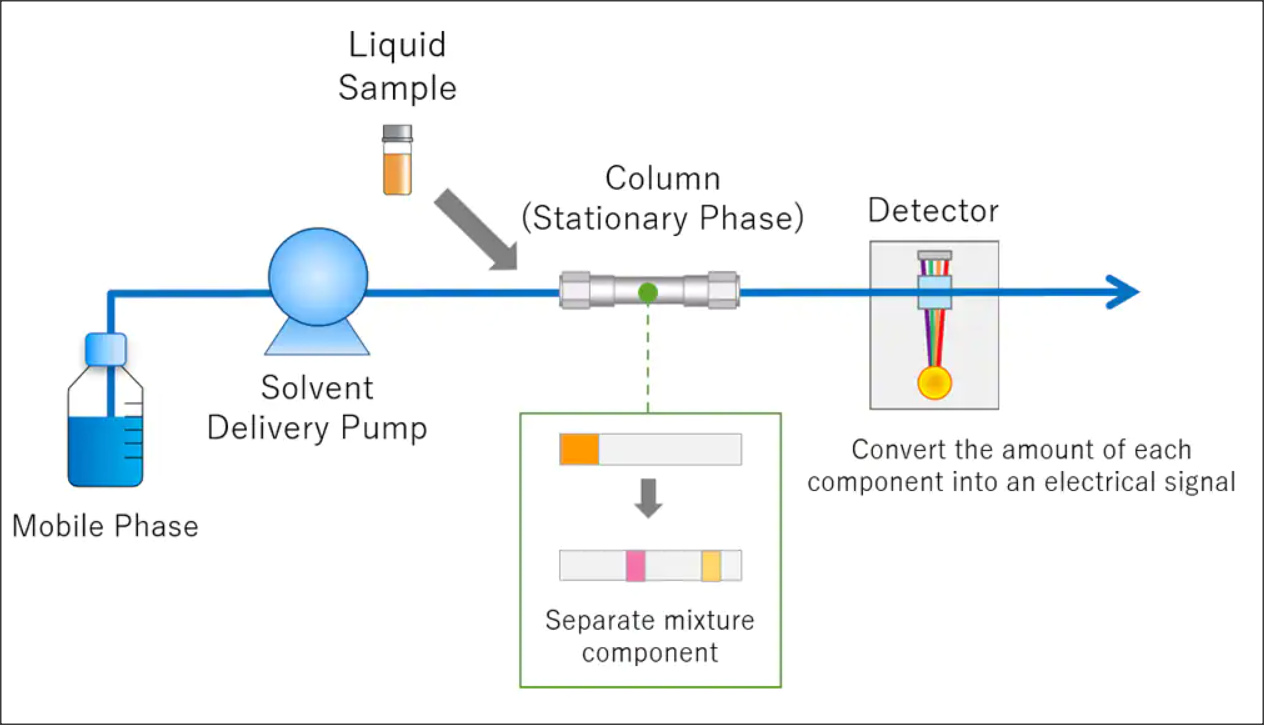
HPLC Calibration
As a well-established HPLC Calibration Company, GL Technologies has emerged as a premier provider of high-quality calibration services for High Performance Liquid Chromatography (HPLC) systems throughout California. With a commitment to precision and accuracy, our expert technicians use state-of-the-art equipment and methodologies to verify that HPLC instruments deliver reliable results for our clients in pharmaceutical, biotechnology, medical, and research industries. We offer comprehensive calibration solutions tailored to meet the unique needs of each customer, adhering to strict regulatory standards while maximizing instrument performance and longevity. As a trusted partner in analytical instrumentation, GL Technologies continues to set the industry standard for proficiency, reliability, and customer satisfaction.


HPLC REPAIR
As a leading HPLC repair company, we pride ourselves on delivering exceptional service and expertise to provide the seamless functioning of your high-performance liquid chromatography systems. With a team of skilled technicians with in-depth knowledge and experience, we offer comprehensive HPLC repair solutions tailored to meet the specific needs of each client. From diagnosing issues to sourcing and replacing components, we prioritize precision to minimize downtime and maximize productivity in your laboratory. Whether it's troubleshooting minor glitches or undertaking major overhauls, trust the GL-Tec HPLC repair specialists to deliver quick, reliable, and cost-effective solutions, allowing you to focus on your research with confidence.