Calibration Services

Whether addressing emergency situations, performing routine maintenance, or installation of new equipment , our customers rely on the expertise and diverse experience that GL Technologies LLC can provide. Our experience performing cGMP compliant calibrations and maintenance has been relied upon by industry leaders in the medical device, biopharmaceutical and life science industries as well as local municipalities and military installations.
We specialize in calibration and troubleshooting of Clean Utilities, Automated Process Equipment, Analytical Laboratory Equipment, HVAC controls, SCADA and Building Management Systems.
Metrology Lab and On-Site Capabilities Include
| Mass | Pressure /Vacuum | Lab Equipment |
|---|---|---|
| Analytical Balances Pan Balances Floor Scales Load Cells 1 mg to 40 kg | Gauges Transmitters Pall Display 30 inHg to 300 psi 0 to 10,000 psi | Autoclaves Depyrogenation Ovens Glassware Washers Bioreactors Stir Plates Timers Centrifuges |
| TEMPERATURE | UTILITY SYSTEMS | PROCESS CONTROLS |
| Fluid Baths -80 to 300℃ Dry Baths -30 to 400℃ Thermometers Liquid in Glass Digital Environmental Chambers Incubators Freezers W/ Digital Displays Refrig. W/ Digital Displays Deli Box W/ Digital Displays Chart Recorders Hygrometers Temp Transmitters | Water For Injections (WFI) Reverse Osmosis / De-Ionized Water (RO/DI) Clean in Place System (CIP) Building Environmental Monitoring System Total Organic Compound Analyzer (TOC) | SMART Transmitters Foundation FieldBus Traditional I/O Transducers InSitu Analyzers SCADA DCS PLC |
Method Development
Whether the scope and application of your proposed method is standard, laboratory-based or non-standard, your organization will be required to develop operating procedures for its consistent execution.
GL Technologies can develop procedures that will allow you to generate data consistent with the scope and application of your chosen method. By establishing quality control procedures, your organization will have an appropriate process in place to generate reliable research data.

Uncertainty Analysis
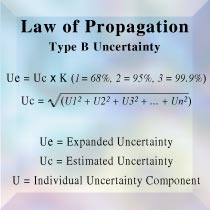
Your laboratory's quality control procedures must be able to monitor the validity of tests and the calibration of your equipment. Only through recorded data with properly calibrated equipment can accurate trend analysis take place.
GL Technologies is your partner in providing quality calibration services. Proper calibration of your equipment keeps your project on track from research, to process development, through to manufacturing.
Enterprise Asset Management
GL Technologies delivers premier asset management services that provide proven financial and time-management benefits. From laboratory equipment purchases through cataloging equipment for future use, our assistance with tracking your calibrated equipment will allow you to monitor your asset budget while developing or manufacturing your product.
GL Technologies can asset tag laboratory and process equipment into a proprietary database and automate notifications when equipment certification or re-certification is due, allowing you to focus your time and energy on research and production.

Program and Procedure Development

Calibration Maintenance Program Development is a critical component of any cGMP Research & Development, Clinical or Production facility.
GL Technologies provides expertise in documenting controlled process Standard Operating Procedures, allowing your organization to focus on its core competencies: research and development, clinical and production operations.
Our procedures and programs provide a detailed and compliant approach to ensure regulatory mandates are addressed upfront.
Personnel Placement
On-site personnel reduce your organization's overall operating cost, increasing quality and productivity. Reduced “escort” time and increased familiarity with your organization's internal programs and policies have proven extremely valuable when headcount is not an option.
GL Technologies has successfully placed short- and long-term on-site personnel in large and small Biopharmaceutical and Medical Device organizations. Our personnel provide services that meet the unique needs of our clients.
We firmly believe in employing staff with integrity and engaging our clients in a quality, no-nonsense manner. Our focus is keeping open lines of communication to ensure the continued efficiency of your organization.

Calibration Services for Regulated Industries
At GL Technologies, we specialize in delivering comprehensive calibration services designed for the strictest industry standards. Our clients in biopharmaceuticals, medical device manufacturing, laboratory research, and other regulated industries rely on us to ensure equipment accuracy, regulatory compliance, and peak operational performance. Whether your lab needs pipette calibration, HPLC system verification, or full-scale preventative maintenance, we provide tailored solutions that keep your operations audit-ready and efficient.
Why Calibration Matters in Regulated Environments
Calibration is more than just a maintenance task—it’s a compliance and quality assurance imperative. In industries where precision is non-negotiable, even minor deviations in instrument accuracy can lead to failed audits, invalid data, or costly production setbacks. GL Technologies helps your team:
Meet FDA, GMP, and ISO 17025 requirements
Maintain accurate and reproducible test results
Reduce downtime and prevent equipment failures
Document traceability for every calibration performed
Our meticulous approach ensures you not only meet regulatory standards but consistently exceed them.
Full-Service Calibration Solutions
GL Technologies provides on-site and off-site calibration services to support your laboratory and production operations. Whether you're working in a cleanroom, research lab, or manufacturing facility, our certified technicians calibrate a wide range of instruments and systems including:
Lab Equipment Calibration
Balances and scales
Pipettes and burettes
Ovens, incubators, and refrigerators
Temperature and humidity chambers
Autoclaves and centrifuges
Thermometers, hygrometers, and pressure gauges
Analytical Instrument Calibration
HPLC systems
GC systems
UV/Vis spectrophotometers
TOC analyzers
pH meters and conductivity sensors
Facility and Utility Systems
RO/DI water systems
Clean steam systems
HVAC sensors and controls
Temperature mapping for storage and production areas
Process control instrumentation
Each calibration includes detailed documentation, certificates with NIST-traceable standards, and optional as-found/as-left data reporting.
Industries We Serve
We focus on supporting clients in highly regulated, mission-critical environments, with deep expertise in:
Biopharmaceutical Manufacturing
Medical Device Production
Biotech and Life Sciences
Clinical and Analytical Laboratories
Food and Beverage Production
Chemical Processing Plants
Our understanding of both equipment function and regulatory expectations makes us a trusted partner in industries where precision, sterility, and safety are paramount.
Compliance-Driven Calibration Protocols
GL Technologies adheres to Good Manufacturing Practices (GMP) and complies with FDA 21 CFR Part 11, ensuring your calibration records are secure, auditable, and electronic-ready. Every service is performed according to documented procedures and validated against the latest compliance frameworks, including:
ISO/IEC 17025
ANSI/NCSL Z540
USP <1058> for analytical instruments
Manufacturer specifications and customer SOPs
Our team is trained in the latest quality standards and audit expectations, helping clients sail through inspections by the FDA, EMA, or third-party auditors.
Preventative Maintenance & Equipment Lifecycle Support
In addition to calibration, GL Technologies offers preventative maintenance services that extend the life of your critical assets. We work proactively to identify early signs of wear, degradation, or drift in performance. Our preventative maintenance plans help reduce unscheduled downtime, save on costly repairs, and ensure consistent output.
When needed, we also provide instrument qualification, re-certification, and system upgrades to keep your lab ahead of evolving technologies and regulations.
On-Site and Off-Site Flexibility
Our services are designed around your operational needs. For clients who require minimal disruption, we offer on-site calibration by our experienced field technicians. For clients with portable equipment or smaller instruments, off-site calibration at our dedicated service center ensures fast turnaround and detailed care.
Whether you operate one lab or manage facilities across the region, our flexible scheduling and nationwide service network ensure your calibration stays on track.
Why Choose GL Technologies?
With more than two decades of experience, GL Technologies is the calibration partner of choice for labs, manufacturers, and cleanrooms across the country. Here’s what sets us apart:
ISO 17025-compliant calibration procedures
Highly trained technicians with deep equipment knowledge
Customized service plans for each client
Electronic calibration reports and full traceability
Rapid response times and exceptional customer service
Seamless integration with your quality management system (QMS)
Our team works hand-in-hand with your internal QA, metrology, and operations teams to deliver peace of mind—and measurable results.
Calibration Certifications and Documentation
Every calibration we perform is accompanied by:
Calibration certificates with NIST traceability
Equipment performance summary (as-found/as-left)
Environmental conditions at time of service
Signature of certified technician
Unique certificate ID for traceability
Digital and hard-copy records available
These records help clients maintain proper documentation for internal audits and regulatory inspections. We can also upload data directly to your CMMS or LIMS platforms upon request.
Schedule Your Calibration Today
Don't let inaccurate instruments or overdue validations put your operations at risk. GL Technologies is ready to deliver efficient, accurate, and compliant calibration services that protect your people, your product, and your reputation.
About GL Technologies
GL Technologies, based in San Diego, is a specialized service provider catering to the highly regulated industries of biopharmaceuticals, pharmaceuticals, medical devices, and government sectors. The company focuses on delivering expert solutions in equipment calibration, validation, and compliance services, ensuring that clients meet stringent GMP (Good Manufacturing Practice) and FDA regulations. GL technologies is a trusted partner from commissioning new plants to decommissioning with compliance. GL can place dedicated motivated quality personnel on site anywhere. A program can be designed or revamped for the customers needs from design of CMMS to SOP development, specification development and performance of calibrations.
With a dedicated team of 29 technicians, GL Technologies offers precision calibration, preventative maintenance, and qualification services for laboratory and production equipment used in critical manufacturing and research processes. The company’s expertise is supporting its clients in maintaining regulatory compliance and operational efficiency.
As a full-service company specializing in equipment calibration, repair, and certification services for biopharmaceutical, pharmaceutical, and medical device industries. Our team has extensive experience working with sPRT calibrations along with CMMS software, HPLC OQ validation, and fume hood certifications. Companies of all sizes rely on our team to implement, maintain, and keep their research and manufacturing processes compliant with regulatory standards. Other specialties include building maintenance systems, and mass spectrometry calibrations. GL Tec specializes in IQ OQ PQ services for clients throughout San Diego, San Francisco, Los Angeles, Orange County, and Riverside!


