Medical Device Test Equipment Calibration
In our commitment to educating industry professionals, GL Technologies is providing this wonderful article we found.
By: Germans Frolov
Medical Device Equipment Calibration is a procedure for detecting and fixing the uncertainties in measurements and bringing them to an acceptable level.
The accuracy of the device has a great deal of importance, as it can seriously affect the diagnostic procedure and endanger patients’ life.
The deviation in output is not necessarily the fault of the device itself, nor can it represent the manufacturer’s incompetence. Most commonly, it results from continuous device usage, which over time affects its internal components, resulting in a deviation from the device standard measurement output.
What is Medical Device Test Equipment Calibration?
There is a difference between medical device test equipment calibration and maintenance activities. Maintenance removes fault in a device, whereas calibration helps to detect the defective instrument that has failed to output values according to the manufacturer’s standard baseline readings.
The calibration activity varies from instrument to instrument. The manufacturer, in most cases, provides a built-in system for performing calibration processes. The manufacturer also recommends calibration frequency, although the user itself can devise its frequency, depending on the instrument application and frequency of use.
Every device has its tolerance limit, as defined by the manufacturer.
If the testing device’s calibration results fall within the tolerance limit, the device can be used further. On the other hand, if the calibration fails the device cannot be used for any process.
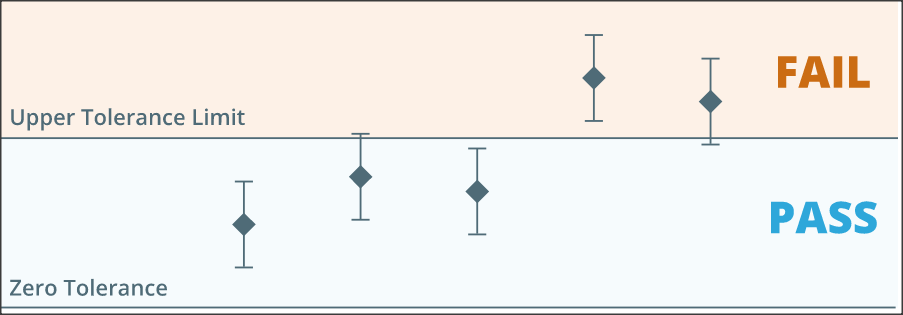
Corrective actions are required for devices that fail the calibration.
Such actions can either be performed in-house or the device can be sent to device specialists. Upon successful corrective actions, the device is again calibrated for verification. If results are satisfactory, the device is allowed to be used again. However, if the defect cannot be fixed, the instrument is permanently damaged and cannot be used further.
The Calibration Cycle is Followed by Complete Documentation
Common Documentation Includes
Calibration Plan, divided into monthly, quarterly, and yearly basis
Calibration steps or a Standard Operating Procedure (SOP)
Calibration Results Sheet indicating the actual results and tolerance limits
Calibration status is also indicated on the device, showing the calibration status, date of calibration, and next due date for calibration.
For example, MRI scanners are regularly calibrated for different sections, including the Imaging Gradients. It allows to keep the imaging output according to standard measurements and helps to accurately detect any changes in tissue structure up to the microscopic level.
Equipment Calibration Related Regulations and Standards
The Medical Device Industry is strictly regulated by regulatory bodies such as Food and Drug Administration (FDA) in the United States to make them compliant with the regulations. Additionally, standardization bodies such as the International Organization for Standardization (ISO) also provide standards for performing medical device calibration.
Common Regulations and Standards for Medical Device Companies
FDA 21 CFR Parts 11
The FDA 21 CFR Part 11 is the FDA’s guidance regarding electronically saved records. Electronic records act as an alternative to manual records. Compliance with this act makes the manufacturer’s electronic records trustworthy.
This regulation also guides electronic signatures, an alternative to manual signatures. However, this regulation does not apply to the electronic records transmitted by electronic means.
FDA 21 CFR Parts 820
The FDA 21 CFR Part 820 is the FDA’s Quality System Regulation for production and process control of medical devices. This regulation allows manufacturers to provide valid inspection and test equipment results by establishing routine calibration procedures.
This regulation also bounds manufacturers to handle all medical devices so as not to harm their accuracy.
Quality System Regulation (QSR)
Quality System Regulation (QSR) is the FDA’s quality policy for medical device manufacturers, similar to the Current Good Manufacturing Practices (cGMP) for Food, Drugs, and Biologics. The QSR only applies to finished medical devices that are ready to be used.
The QSR does not target any particular medical device but provides flexible guidelines to cover all medical devices. QSR puts the responsibility on the manufacturer to develop and establish their quality policies in light of QSR guidelines.
ISO 13485:2016
ISO 13485 is a standard for medical device manufacturers developed by the International Organization for Standardization (ISO). It was reviewed and updated in the year 2016. The number 2016 comes with its terminology.
ISO 13485:2016 is the set of requirements for a Quality Management System (QMS) in the medical device industry.
This standard covers all life cycle stages of the medical device, including manufacturing, storage, distribution, installation, and servicing.
Recommended Reading: Medical Device Quality Management System (QMS)
Why is Medical Device Test Equipment Calibration Important?
Medical devices can drift away from the measurements due to their internal components. This phenomenon is common among all manufacturers, even with the most advanced ones. Over time, the internal components – both mechanical and electronics wear and tear. As a result, their performance starts to decrease and results in erratic readings. These erratic readings can directly affect the patient life, such as in the case of ultrasound units.
Secondly, as mentioned above, regulatory bodies such as the FDA in the US have made it mandatory for the manufacturers to perform routine calibration and maintain calibration records. Otherwise, the manufacturer is not granted accreditation and is not allowed to start or continue its operations.
The calibration process is necessary to detect these erratic readings and comply with the regional regulatory bodies. The calibration must be regularly performed, and documentation maintained for presenting during a routine inspection.
Medical Equipment Calibration Requirements
The calibration is critical in the medical device industry and must be performed according to regulatory requirements.
Considering its importance and significance, manufacturers prefer to dedicate separate resources for calibration activity such as:
Establishing a department
Hiring skilled personnel
Implementing an Equipment Calibration Management Software
Maintaining instruments to perform calibration
Common requirements for calibration, as per the FDA 21 CFR Parts 820, section – 820.72 “Inspection, measuring, and test equipment”, are summarized below.
Procedures and Instructions
It is the manufacturer’s responsibility to develop procedures for regular calibration of each device. The procedure should contain tolerance limits that help in qualifying instruments (fail or pass). In case the device calibration fails, the procedures should guide how to meet the acceptable limit.
Secondly, procedures should document what to do if the device completely fails the calibration.
For example, if a department replaces a device due to fault, it cannot use the new device without calibration and verification from relevant departments such as the Quality Assurance Department. In this scenario, the procedure for a new device calibration is required and should be developed by the device user department. The procedure should also contain steps to replace and update the relevant documents.
Regulatory Standards
This includes the applicable standards for performing routine equipment calibration. The calibration standards should be traceable to national and international standards such as the ISO 13485:2016.
In the absence of available standards, the manufacturer shall establish its own, in-house standards.
Documentation and Records
The calibration activities must be documented and calibrations record well-maintained. A proper calibration plan should be in place, indicating each device’s calibration frequency, i.e., weekly, monthly, quarterly, or yearly. All the calibration records should be readily available during an inspection.
The calibration records should contain the following information:
Equipment identification number
The date when calibration was performed
Name of the person performing the calibration
The due date for the next calibration
In addition to records, the calibration status or tag should be indicated on the individual device, including the calibration date, the next calibration due date, and the name of the person performing the calibration.
A software solution such as SimplerQMS can help you schedule each device’s calibration frequency and remind you when it’s time for the next calibration.
The software is 21 CFR Part 11 compliant and allows you to sign calibration records with 21 CFR Part 11 compliant electronic signatures.
Training of Personnel
These include training methods for the personnel involved in calibration activities, including the instructions on how to handle and store the calibrated devices.
The manufacturer is responsible for conducting training for the personnel involved in calibration activities. Therefore, a proper training calendar and a training plan must be in place.
All training activities must be recorded and maintained, including the topic details, attendance sheet, and trainer details.
For example, the training plan for the instrument calibration can include the following:
The personnel must be trained to use the master instrument according to standard specifications.
The personnel must understand the instrument’s operational requirements, such as power or utility for normal operation.
The personnel must be trained to establish a safe and secure connection between the master and calibrating instrument.
The personnel must be trained about the device being calibrated. It includes operational training, available functions and features of the device, and how it connects to the master instrument.
The personnel must be trained about the relevant applicable standards and their requirements.
The personnel must be trained to complete all the documentation correctly. It includes recording the observed values against the standard values, identifying tolerance levels. The personnel must indicate calibration status on the device according to approved formats such as tags.
How to Manage Medical Equipment Calibration Effectively
As mentioned above, calibration is of utmost importance for manufacturers when meeting compliance requirements. Regulatory bodies rely on the documentation developed by the manufacturer to decide whether or not the manufacturer has effectively established and followed calibration procedures.
First of all, all the documents should be readily available for inspection by the regulatory bodies. If not produced timely, the regulatory body can assume that the calibration was not performed. The documents should be up-to-date and follow the approved calibration plan. The records for defective devices should not be included, and they must be separated from the supported devices.
An effective way of managing calibration activities is by using digital solutions such as Equipment Calibration Management Software by SimplerQMS. SimplerQMS software solution is compliant with FDA 21 CFR Parts 11, FDA 21 CFR Parts 820, Quality System Regulation (QSR), ISO 13485:2016.
It allows users to store all the calibration documents in a centralized system, in secured cloud-based storage. The system tracks the documentation status and automatically prompts email alerts to the responsible person to complete assignments.
Equipment Calibration Software by SimplerQMS allows you to create a calibration plan of the device, so you don’t need to inspect device by device ‘on the floor’ to plan the device calibration. The software automatically creates a complete equipment calibration log that can easily be presented during audits or inspections.
The system follows the calibration schedule and automatically alerts the responsible person when calibration is due for a particular device. This prevents you from missing the calibration for a particular device, as missed calibration can seriously cause regulatory non-conformance.
To see Equipment Calibration Management Software by SimplerQMS in action and learn how it can help your organization achieve efficiency book a free demo!
Final Thoughts
Calibration is becoming an important tool for the regulatory bodies to gauge medical device manufacturer compliance adherence. It sometimes becomes difficult to track calibration of all devices, for example, due to human negligence error.
In large manufacturing facilities, some devices are left without their documentation, although they have undergone proper calibration procedures. Sometimes due to equipment or area shifts, device identification is not updated.
All the problems mentioned above can be effectively solved by using digital tools like the SimplerQMS Calibration Management Software. It helps the manufacturer to adhere to regulatory body requirements streamline various calibration processes.
About GL Technologies
GL Technologies (formally Calibration Consultants) is a full service calibration provider specializing in the Biopharmaceutical and Medical Device industries. Our management has worked in, managed, and developed FDA cGMP fully compliant programs. Our services include roving crews for the Biopharmaceuticals for regular scheduled calibrations or shutdown and new system situations.
Original Source: simplerqms.com


